Yield and Brix increases of sugarcane (Saccharum sp.) with organic beneficial soil microbes after reducing chemical nitrogen fertilizer (observational pilot trial in South Africa)

Written by M.A. Young and R. Saunders
Figure 1. Overhead photograph of an irrigated plot (100 hectares) of sugarcane (Saccharum sp.) treated with synthetic nitrogen fertilizer at the recommended application rate of 160 kg/hectare (Control 1, 2 3) and ExploGrow™ at 6 L/ha + 90 kg/ha of synthetic nitrogen fertilizer (44% nitrogen reduction).
Photo provided by Jonathan Bucke.
Summary of Senekal Familie Boerdery ExploGrow™ trial
- Mkuze, the largest private sugarcane farm in the world
- Senekal irrigates 4,000 of the 24,800 hectares under cane
- Irrigated sugarcane incurs large electrical costs
- Sugarcane without irrigation is influenced by environmental stress such as drought, heat exposure, pests and diseases often inhibiting crop growth
- Requires continued innovation and experimentation with farming program
- ExploGrow™ biological fertilizer to improve growth, yield and quality of sugarcane
- Reduce the need for synthetic fertilizer and irrigation
Land 701: Sugarcane farmer's chemical fertilizer program (Control)
- Nitrogen applied: 160kg per hectare
- Yield: 86 tons/ha
- Pre-harvest Brix: 21
Land 702, ExploGrow™ organic biological fertiliser + Sugarcane farmer's chemical fertilizer program
- N-P-K reduced -44% to 90-0-0 + 6L/ha* ExploGrow™
- Yield: 114 tons/ha (32.6% increase in yield)
- Pre-harvest Brix: 25 (18.9% increase in Brix)
- Increased biodiversity of soil, improving the health and productivity
- Increased root size, weight and length
- Increased leaf mass, size, chlorophyll fluorescence and photosynthesis
*[recommended application rate for Sugarcane crops 8-10 liters per hectare]
Outline
- Introduction
- Methods - ExploGrow™ biofertilizer application
- Results
- 3.1 Yield
- 3.2 Sugar content (Brix)
- 3.3 Soil microbial analysis
- 3.4 Soil and root development
- 3.5 Enhanced drought stress tolerance: An observation
- 3.6 Increased leaf mass and leaf photosynthesis
4. Conclusion
5. Acknowledgements
6. References
1. Introduction
Plants are susceptible to environmental (abiotic) stress every day that can often inhibit plant growth in some way (Wang et al. 2003). As time goes on, environmental stress either passes or the plant will have to accommodate and adapt to overcome the stress through natural biological processes. There is a natural flux between times of permitted plant growth and inhibited plant growth. Plants are capable of handling the continuous change in growth rates by physiologically acclimating themselves to the changing environment around them (Peleg et al. 2011). Plants activate cellular responses when experiencing abiotic stress and are capable of producing and regulating a wide range of hormones and proteins, including plant defence proteins that can protect the plant (Peleg et al. 2011; Wang et al. 2003). At the same time, soils naturally have a coping mechanism for times of environmental stress and respond through the vigour of abundant microorganisms that can either promote or inhibit plant growth (Glick 2012). Among the plethora of soil microbes, plant growth promoting bacteria (PGPB) are capable of facilitating plant growth during times of environmental stress, such as heat exposure, drought, excess rain, insect damage, toxins and disease (Glick 2012; Kang et al. 2010). There are several varieties of beneficial fungi and bacteria that naturally occur within the soil, however are in very low quantities and thus, are unable to maintain the scale of biological processes required by commercial farming practices (Glick 2012).
The Senekal Familie Boerdery have approximately 20% of their 24 800 hectares of sugarcane under irrigation. Sugarcane under irrigation is less susceptible to environmental stress, however the trade off for increased irrigation incurs large electrical costs. The remaining 20 800 hectares of sugarcane is not irrigated, thus is more susceptible to environmental stress which further decreases the potential yield of the crop. The collaboration between Senekal Familie Boerdery and ExploGrow™ aims to improve the health, biodiversity and productivity of the soil, increase the yield and sugar content (Brix) of the sugarcane crop, while simultaneously reducing the need for synthetic fertilizer and increased irrigation.
2. Methods - ExploGrow™ biofertilizer application
ExploGrow™ contains a wide variety of PGPBs that encompass many different roles in the soil, including rhizobia microbes, Azospirillum, Enterobacteria, Azorhizophillus, Sinorhizobium and Azotobacter. These rhizobia species play vital roles in the rhizosphere and rhizoplane, bonding within roots and on root hairs, notably improving nutrient uptake (Emtiazi et al. 2007; Glick 2012). Nitrogen fixing microbes are responsible for fixing nitrogen into approximately 65-70% of the world’s soil each year, with nutrient deficient soils uptaking the greatest amount of atmospheric nitrogen (Rashid et al. 2008). Rhizobia are capable of fixing between 100 and 300 kg of nitrogen into the soil each year, and in exchange, enter into a symbiotic relationship with the surrounding plants through utilizing plant sugars and protection (for both plant and microbe) within the rhizosphere (Reid 2005).
ExploGrow’s Bacillus and Psuedomonas species play essential roles in nutrient recycling in the soil. The microbes are responsible for decomposing organic material into simpler constituents including, mineral nutrients, plant hormones and natural antibodies to aid plant growth and suppress potential pathogens or physical threats (Glick 2012). Phosphorus and iron are among many nutrients locked in an insoluble form within the soil and are thus not accessible for plant uptake (Glick 2012; Khan et al 2007). As solubilization of these minerals is an extremely energy intensive process, plants are unable to harness the essential nutrients vital to sustaining healthy plant growth. Not only can Bacillus and Psuedomonas species solubilize vital nutrients and supply them to plants through long chains of mycelium networks, they can further bioremediate polyaromatic hydrocarbons among other toxins into non toxic substances, subsequently healing the surrounding soil (Glick 2012; Khan et al 2007; Queiroga et al. 2003).
A 100 hectare plot was divided into four 25 hectare quarters at Mkuze private sugarcane farm. Three of the 25 hectare quarters (controls 1, 2 and 3) were treated with traditional farming techniques using 160 kg/ha of synthetic nitrogen. The fourth quarter (ExploGrow™) was treated with 90 kg/ha of synthetic nitrogen fertilizer (44% less) and 6L/ha of ExploGrow™ biofertilizer.
- Sugarcane ratoons planted: December 2016
- Sugarcane harvested: November 2017
- Nitrogen treatments (25 ha/plots):
Controls: 160 kg/ha synthetic nitrogen fertilizer (application at time of planting)
ExploGrow™: 6 L/ha combined with 90 kg/ha synthetic nitrogen fertilizer (saving 44% N fertilizer)

Figure 2. Aerial photograph (850 ft) of the 100 hectare study site. Control treated (160 kg/ha synthetic nitrogen) sugarcane (Saccharum sp.) on the left, with ExploGrow™ + 90 kg/ha synthetic nitrogen treated sugarcane on the right. Visible differences in sugarcane quality can be seen between the control plot and the plot treated with ExploGrow™.
3. Results
The sugarcane crop treated with ExploGrow™ and 44% less synthetic nitrogen fertilizer produced 32.6% more yield and a higher Brix content of 18.9% than the control sugarcane crop treated with synthetic nitrogen fertilizer only. The increased productivity and quality in the sugarcane treated with ExploGrow™ is closely correlated to the increase seen in the soil microbial count (colony forming units; CFU), the size, mass and length of both roots and leaves; as well as increases in recorded leaf chlorophyll fluorescence and photosynthesis.

Figure 3. Randomly selected sugarcane harvested from profile holes within the 100 ha study site (Saccharum sp.) being washed and prepared for measurements to be collected. Note: differences in root size and development visible between control (160 kg/ha synthetic nitrogen fertilizer) sugarcane and ExploGrow™ (90 kg/ha synthetic fertilizer + 6 L/ha ExploGrow™ biological fertilizer) treated sugarcane after 4 months.
3.1 Yield
- The control area with 160 kg/ha nitrogen, produced 86 tons/ha
- The ExploGrow™ area with 6 L/ha and 90 kg/ha nitrogen, produced 114 tons/ha (an increase of 32.6% in yield)
"The unique microbial composition places ExploGrow™ in a league all of its own". Dr. Malherbe, BSc; BSc Hons.; MSc (Microbiology); Pr.Sci.Nat. (Agricultural Science); PhD (Agronomy)
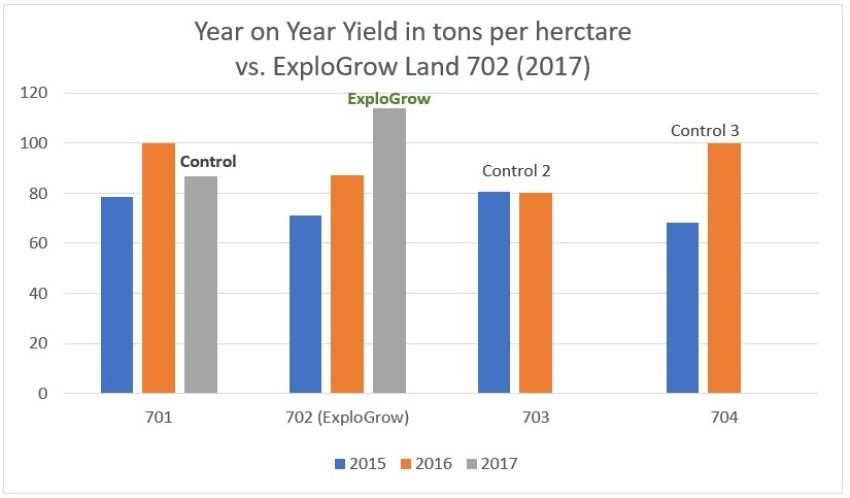
Figure 4. Year on year yield comparisons from the 100 ha plot divided into four 25 ha quarters (land 701; 702*; 703 & 704)
* [Land 702 received ExploGrow™ in 2017 only. Note: The sugarcane plot treated with ExploGrow™ in 2017 had the highest yield in the 100 ha study site over the past 3 years].
3.2 Sugar content (Brix)
Several random sugarcane internodes we harvested from profile holes within the 100 ha study site and the sugar content (Brix) was measured between control and ExploGrow™ treated sugarcane using a refractometer, 7 days before harvest.

Figure 5. Refractometer displaying a Brix of over 30% from a sample of sugarcane treated with ExploGrow™ biofertilizer.
- ExploGrow™ average Brix: 25.08 (from 24 internode samples)
- Control average Brix: 21.08 (from 12 internode samples)
- Brix increase: 4.0 = +18.9% increase
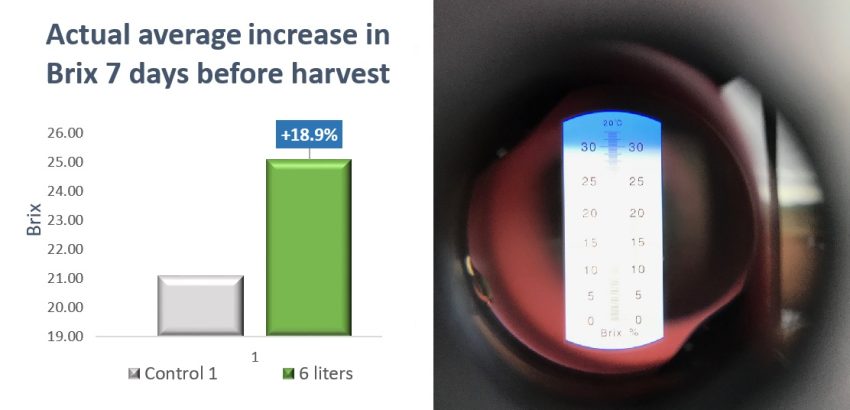
Figure 6. Brix content between control and ExploGrow™ treated sugarcane from the 100 ha study site.
Sugarcane treated with ExloGrow™ demonstrated an increase in Brix quantity, which corresponds with similar studies where applications of biofertilizers were found to also increase the Brix content in sugarcane (Santos et al. 2010; Stamford et al. 2015). This demonstrates the advantage of using biofertilizers in sugarcane farming, where the health of the soil subsequently improves and essential nutrients, hormones and antibodies, become more readily available to the plant.
3.3 Soil microbial analysis
Soil samples were taken from random profile holes within the 100 ha study site in September 2016 before ExploGrow™ was applied and in February 2018, one year after ExploGrow™ was applied. Microbial analyses were conducted between soil samples from September 2016 and February 2018.
Compared to the previous year, the soil treated with ExploGrow™:
- Had 80x more bacteria at 2.0 x 107 CFU
- Had 50x more fungi at 1.0 x 105 CFU
Microbial soil analysis was conducted at Rhodes University by Professor Joanna Dames, at the Department of Biochemistry, Microbiology and Biotechnology. The analysis provides a microbial count per gram of soil, however no identification of the individual species of microorganisms have been made, apart from separation into broad bacterial and fungal categories. Replicate soil samples were serially diluted, plated onto appropriate media (agar gel), incubated and assessed for colony forming units (CFU).
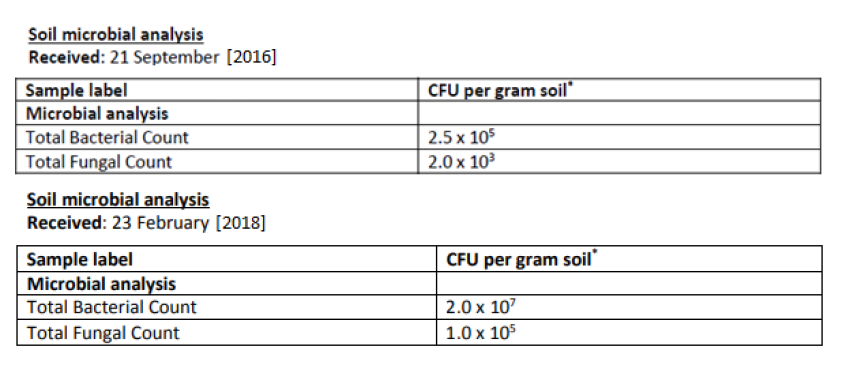
Figure 7. Soil microbial analysis collected from pooled soil samples from the sugarcane (Saccharum sp.) study site demonstrating colony forming units (CFU) of the total quantity of bacterial and fungal counts in the soil before and after the application of ExploGrow™.
3.4 Soil and root development
Five randomly selected clusters of sugarcane were taken from profile holes within the 100 ha study site and assessed for root development and quality between control and ExploGrow™ treated plants, 4 months after the initial application of biofertilizer.
Compared to controls, the root systems treated with ExploGrow™:
- Were more than double in size
- Were more dense
- Penetrated the clay soil more than twice the depth
"The doubling of the root system and the huge amount of Nitrogen activity is just unbelievable!... ExploGrow™ is truly a game changer" Mr. Dreyer Senekal – the recognised Sugarcane lead farmer - of Senekal Familie Boerdery.
Application of biofertilizers, such as ExploGrow™, enhance the plant’s ability to absorb essential nutrients, further reducing their dependence on chemical fertilizers (Glick 2012). Biofertilizers also reduce plant water requirements by ~20%, increase the resistance of plants to extreme heat, cold and frost (through absorption of more minerals), and can typically increase the growth, yield and Brix dramatically (Glick 2012; Stamford et al. 2015).


Figure 8. Comparison of control sugarcane (Saccharum sp.) roots (160 kg/ha nitrogen) with sugarcane roots which had a 44% reduction in nitrogen fertilizer (90 kg/ha nitrogen) and were treated with ExploGrow™ at 6L/ha on the left. On the right, healthy looking soil and roots in the profile hole of the sugarcane treated with ExploGrow™ at 6L/ha.
"This is due to the increased biological activity by the 17 microbes in ExploGrow™ and the consequent much larger root systems" Mark A. Young, BSc.
Compared to the controls, ExploGrow™ treated plots displayed visibly healthier looking soil. The plant growth promoting bacteria that make up ExploGrow™ live within the rhizoplane and rhizosphere of the soil. ExploGrow™ contains Bacillus sp. and Psuedomonas sp. which are responsible for improving the health of the soil by reallocating nutrients, hormones and antibodies, making them more readily available for the plant (Glick, 2012). Over time, ExploGrow™ microbes will break down organic material producing more microbes, and humic and non-humic compounds. These humic and non-humic compounds can potentially improve the color, texture, smell, and feel of the soil; further improving crop development and growth (Magdoff & Van Es, 2010).
3.5 Enhanced drought stress tolerance:
An observation After the sample sugarcane plants were removed from the soil, initial observations were collected on the moisture retention and quality of the treated leaves. The ExploGrow™ treated leaves appeared to retain moisture for a longer period (4 hours +) compared to the sugarcane leaves of the control plants where leaves were noticeably more wilted.

Figure 9. Control (160 kg/ha synthetic nitrogen) sugarcane roots (left, bottom) compared to ExploGrow™ treated roots (right, bottom) 4 hours after being removed from the soil. Leaves were noticeably more wilted on the control sugarcane (left, top) compared to the ExploGrow™ treated leaves (right, top) indicating a lesser affinity to hold onto cellular water in the controls.
Vurukonda et al (2015) suggest that drought is one of the most common environmental stresses in the agriculture industry globally, and often inhibits plant growth and production. Drought has a negative economical effect globally that is felt by farmers worldwide. Innovation is critical and PGPB’s could potentially play a crucial role in promoting plant growth during times of environmental stress. Azospirillum sp. and Rhizobium sp. have displayed the potential to improve the drought tolerance of plants through production of trehalose, protecting the plant against temperature extremes, salt and lack of water (Glick, 2012). ExploGrow™ contains a mixture of PGPB species, thus has the potential to improve the drought resistance of plants and based on the initial observations seen in this trial, additional research is warranted in this field.
3.6 Increased leaf mass and leaf photosynthesis:
Sugarcane treated with ExploGrow™ had noticeably longer and greener leaves compared to control sugarcane treated with synthetic nitrogen (160 kg/ha) after 4 months. Leaves that are longer and greener undergo increased photosynthesis (Brihane et al. 2012), suggesting leaves will continue to demonstrate increased growth over the remainder of the plant life cycle. Chlorophyll fluorescence is used as an indicator of improved leaf photosynthesis taking place in the plant, from enhanced activity of beneficial plant microbes (Brihane et al. 2012). The average chlorophyll fluorescence in leaves treated with ExploGrow™ demonstrated a 46.1% increase compared to controls. The increase in chlorophyll fluorescence in ExploGrow™ treated plants, correlated with a 32.6% increase in sugarcane yield at the end of the harvest period. This suggests the microbes within ExploGrow™ may be responsible for the increase seen in leaf mass and photosynthesis of treated sugarcane.

Figure 10. Marguerite Wescott, MSc, PhD Candidate, Plant Science at the (University Free State, South Africa) collecting chlorophyll fluorescence measurements of control (160 kg/ha synthetic nitrogen) and treated (ExploGrow™) sugarcane (Saccharum sp.) using the Pocket PEA (Hansatech Instruments).
4. Conclusion
The sugarcane (Saccharum sp.) treated with ExploGrow™ and 44% less synthetic nitrogen fertilizer had an increased yield and Brix content, soil bacterial and fungal count, leaf chlorophyll fluorescence and photosynthetic rate. The improvements seen in soil and plant health can be closely correlated to the power of abundant microorganisms available in ExploGrow™.
ExploGrow™ is not only capable of revitalising a nutrient deficient soil but it can also recycle and resupply plants with abundant nutrients, plant hormones and natural antibodies derived from natural (e.g. compost, bedrock and mulch) and unnatural sources (e.g. chemical fertilizer, pesticides and poisons). ExploGrow™ contains microbes that can reactivate soil health by reallocating nutrients, hormones and antibodies in the soil, making them more easily accessible for plant uptake. Furthermore, ExploGrow™ is capable of reversing soil damage from chemical and pesticide use, through biologically remediating and returning the soil to its original and improved healthier state.
In conclusion, ExploGrow™ recommends reducing chemical fertilizer use by at least 50% because our microbes prefer an environment low in nutrients and high in organic matter. ExploGrow™ microbes want to heal, replenish and feed the soil and plants with naturally occurring nutrients, hormones and antibodies derived from the atmosphere, organic material, insoluble compounds, compost, mulch and potentially toxic compounds. The symbiotic relationship ExploGrow™ microbes have with plants is at the molecular scale, offering a service we can’t even begin to see and imagine.
More statistical data available from around 1,500 canes: Indicating increased length of cane, internode counts, remarkable thermal heat mapping observations etc.
5. Acknowledgments
We thank Mr. Dreyer Senekal, Mr. Danie Willemse and the farming team at Senekal Familie Boerdery for their assistance in conducting the field trial, Dr. Joanna Dames for microbial analyses (Rhodes University) and Aerial photos were provided by Jonathan Bucke (Air Wing).
Funding was provided by ExploGrow™ and Senekal Familie Boerdery, Carbon Fertilizer Technologies (Carbotech® Liquid Carbon), Afran Ammonia (Keystone Calcium)
6. References
Birhane, E., Sterck, FJ., Fetene, M., Bongers, F., & Kuyper, TW. 2012. Arbuscular mycorrhizal fungi enhance photosynthesis, water use efficiency, and growth of frankincense seedlings under pulsed water availability conditions. Oecologia. 169(4): 895-904.
Emtiazi, G., M. Pooyan and M. Shamalnasab, 2007. Cellulase Activities in Nitrogen Fixing Paenibacillus isolated from soil in N-free media. World J of Agric Sci. 3: 602-608.
Glick BR. 2012. Plant growth promoting bacteria: Mechanisms and applications. Scientifica. http://dx.doi.org/10.6064/2012/963401.
Kang B, Kim W, Yun H, Chang S. 2010. Use of plant growth-promoting rhizobacteria to control stress responses of plant roots. Plant Biotechnol Rep. 4(3): 179-183.
Magdoff, F., & Van Es, H. 2010. Building Soils for Better Crops, 3rd edition. Sustainable Agriculture Network. Beltsville, MD. ISBN 978-1-888626-13-1.
Peleg Z, Blumwald E. 2011. Hormone balance and abiotic stress tolerance in crop plants. Current Opinion in Plant Biology, https://doi.org/10.1016/j.pbi.2011.02.001.
Queiroga CL, Nascimento LR, Serra GE. 2003. Evaluation of paraffins biodegradation and biosurfactant production by Bacillus subtilis in the presence of crude oil. Braz. J. Microbiol. 34(4): ISSN 1517-8382.
Rashid HK, Mohiuddin MD, Rahman M. 2008. Enumeration, isolation and identification of nitrogen fixing bacterial strains at seedling stage in rhizosphere of rice grown in non-calcareous grey floodplain soil of Bangladesh. J Fac Environ Sci Technol. 13: 97-101.
Reid G. 2005. Soil biology basics: Microbes and minerals. Profitable and Sustainable Primary Industries, State of New South Wales, AU. Retrieved from http://www.dpi.nsw.gov.au/__da...
Khan, MS., Zaidi, A., Wani, PA. 2007. Role of phosphate solubilizing microorganisms in sustainable agriculture: a review. Agronomy for Sustainable Development. 27(1): 29-43.
Santos, DH., Tiritan, CS., Foloni, JSS., Fabris, LB. 2010. Sugarcane yield under mud cake addition enriched with soluble phosphate. Tropical Agricultural Research. 40: 454-461.
Stamford, NP., Neto, DES., De Freitas, ADS., Oliveira, ECA. 2015. Rock biofertilizer and earthworm compost on sugarcane performance and soil attributes in two consecutive years. Scientia Agricola. 73: 29-33.
Vurukonda, SKP., Vardharajula, S., Shrivastava, M., Skz, A. 2016. Enhancement of drought stress tolerance in crops by growth promoting rhizobacteria. Microbiological Research. 186: 13-24.
Wang W, Vinocur B, Altman A. 2003. Plant responses to drought, salinity, and extreme temperatures: towards genetic engineering for stress tolerance. Planta. 218: 1-14.
Additional information:
Egamberdieva, D. and Z. Kucharova, 2008. Cropping effects on microbial population and nitrogenase activity in saline arid soil. Turk. J. Biol., 32: 85-90.
Kim BK, Chung JH, Kim SY, Jeong H, Kang SG, Kwon SK, Lee CH, Song JY, Yu DS, Ryu CM, Kima JF. 2012. Genome Sequence of the Leaf-Colonizing Bacterium Bacillus sp. Strain 5B6, Isolated from a Cherry Tree. J Bacteriol., 194(14): 3758-9, doi: 10.1128/JB.00682-12.
De Vrieze J. 2015. The littlest farmhands. Science, 349 (6249): 680–683.



